Pancreatic Enzymes Continuous Tube Feeding How Often to Dose
Individuals with pancreatic insufficiency (PI) as a result of conditions such as cystic fibrosis (CF) require pancreatic enzyme replacement therapy (PERT) to assist macro and micronutrient digestion and absorption. Patients with PI receiving enteral feeds require PERT to digest their enteral formula. Standard polymeric feeds administered with PERT have been shown to be well tolerated and absorbed and are generally the first-line option for enteral feeding in CF [1]. An elemental formula high in medium chain triglycerides (MCT) may be an alternative if a patient continues to show signs of malabsorption or poor growth despite administering PERT or if no options for PERT administration are appropriate [1]. One study however demonstrated CF infants on a MCT-based formula had improved fat absorption with PERT vs no PERT, suggesting that even with high MCT elemental feeds, addition of PERT may be optimal [2]. There are several options for dosing enzymes with enteral feeds. The method chosen will depend on the feeding regimen (i.e. continuous or bolus feeds), the patient's ability to take enzymes orally and the size and type of the patient's enteral feeding tube. According to current national guidelines [1], PERT should be taken orally with enteral feeds wherever possible with the dose based on fat content of the enteral formula. If oral administration is not possible, there are two previously described alternatives. Ferrie et al. [3] describe dissolving crushed or whole enzyme beads in sodium bicarbonate to form an activated solution ready to be delivered directly into the feeding tube or mixed into the enteral formula. Alternately, the whole enzyme beads can be suspended in thickened fruit juice or pureed baby food and administered directly into the feeding tube [3, 4]. Crushing enzyme beads and adding directly to enteral formula has not been previously detailed in the literature. We describe the case of an extremely premature infant with CF where this method proved to be effective when alternative methods were contraindicated. It should be noted that a novel in-line digestive cartridge containing immobilized lipase [5], which digests fat as the enteral feed passes through the cartridge, is available in North America but not internationally. This is a useful option for PERT with enteral feeds where it is available.
Extremely premature (24-week gestation) female dichorionic diamniotic twins diagnosed with F508del homozygous CF on newborn screening were referred to our CF team from the neonatal intensive care unit at our CF centre [6, 7]. Twin 2 (birthweight 660 g) passed away on day 40 after withdrawal of intensive care due to respiratory failure and significant neonatal complications. Twin 1 (birthweight 510 g, birth length 29 cm) also had a difficult neonatal course, requiring ventilator support for 60 days before extubation to CPAP, and an initial period of total parenteral nutrition (TPN). Enteral feeds of breast milk with human milk fortifier (HMF) were introduced at 26.5-week corrected gestational age (CGA) via 5-Fr NGT. By 28-weeks CGA, twin 1 was weaned from TPN to full continuous nasogastric feeds of breast milk with additions of HMF and protein powder. PERT was commenced at 30-weeks CGA due to insufficient weight gain and clinical symptoms of malabsorption (including fat globules on stool microscopy) despite enteral feeds providing 100% estimated energy requirements (EER). The patient was unable to take enzymes orally as she was intubated and ventilated, and had immature oral skills. Dissolution of enzymes in sodium bicarbonate was contraindicated as the patient was being treated intermittently with sodium bicarbonate for metabolic acidosis. Use of thickened juice has only been reported to work in 8-Fr tubes or larger and it was considered likely this solution would clog the 5-Fr NGT [2]. It was also not considered appropriate to introduce fruit juice to the gut of an extremely premature infant.
Crushing enzyme beads and adding to the feed before delivery into the stomach via NGT was considered. This method was trialled because no other option of enzyme administration was practically or medically suitable, and this enabled the patient to continue to receive breast milk in preference to switching to an elemental, MCT-based formula. With this method, the enzymes are activated in the feed resulting in a "pre-digested" formulation, which negates the need to keep the outer enteric coating on the enzyme beads intact when they reach the stomach and are exposed to gastric acid. During the neonatal period, the patient received breast milk with additions of HMF, MCT oil and protein powder (to provide 420 kJ/100 ml) to meet her high energy and protein requirements of up to 130% EER. Her enzyme dose was calculated on the fat content of the feeds, providing around the maximum recommended dose of 10,000 IU/kg/day [1]. A reduction in clinical symptoms of steatorrhoea and reduced fat globules on stool microscopy were observed with PERT commencement and with increases in PERT dose when malabsorptive symptoms or poor weight gain recurred.
Liquid formulations of sodium chloride and fat soluble vitamins were commenced at around the same time as PERT in line with national CF recommendations [1]. A proton pump inhibitor was commenced at around 3-weeks CGA for clinically evident reflux. From 1-month CGA, she was transitioned to high calorie (420 kJ/100 ml) polymeric infant formula as breast milk supply weaned. Bolus feeds were tried multiple times during the neonatal period but were not tolerated until around 2-months CGA.
The patient described remained in hospital until 7-months CGA. The patient's weight and length percentile charts from the neonatal period can be seen in Fig. 1. Weight and length percentiles from term to 7-month CGA can be seen in Fig. 2. From commencement of PERT to discharge at 7-months CGA, the patient received only pre-digested feeds (with the addition of crushed enzymes) via NGT (5–8-Fr) with no resulting tube blockages. From around 2-months CGA until discharge at 7-months CGA, the patient was able to achieve appropriate catch-up weight gain on bolus feeds of polymeric infant formula providing 80–100% normal EER for age. Poor feed tolerance with gagging, retching and vomiting was a complication from around 4-months CGA which contributed to significant oral aversion, in turn impeding progression to oral feeding or PERT. The patient was discharged from hospital at 7-months CGA and eventually progressed to gastrostomy and Nissen's fundoplication at 3 years CGA due to ongoing oral aversion and significant reflux and vomiting.
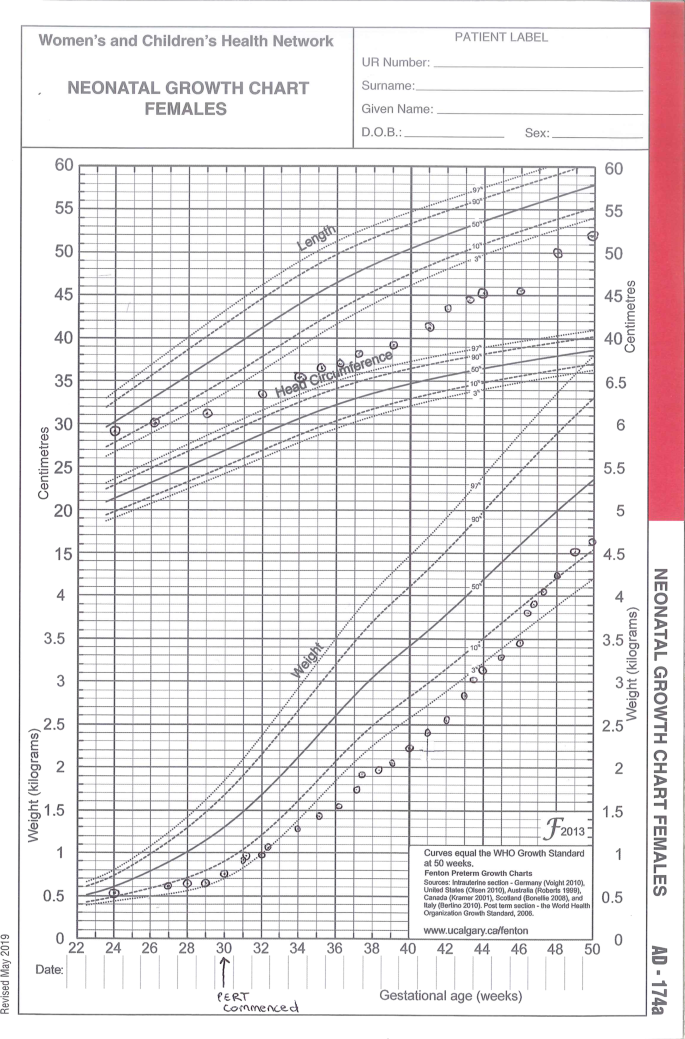
Weights and lengths from birth to 50-weeks Corrected Gestational Age (CGA) (Fenton preterm growth charts).
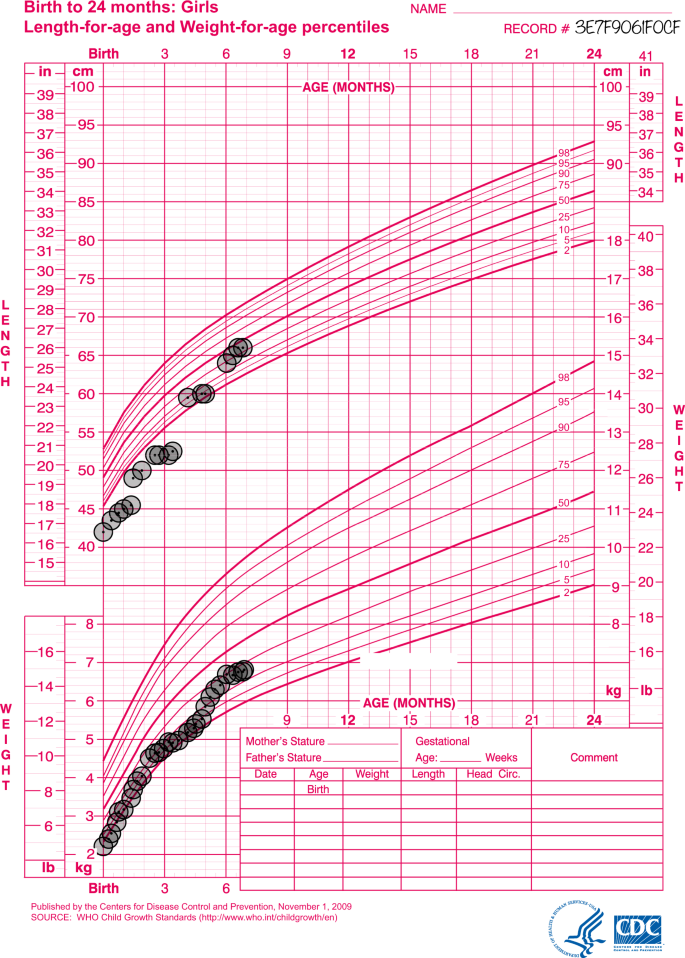
Weights and lengths from term to 7-months Corrected Gestational Age (CGA) (WHO Child Growth Standards).
This case outlines the successful implementation of an alternative method of administering PERT for patients receiving enteral feeds. The addition of crushed enzyme beads to pre-digest enteral formula has been shown to be safe and effective in this patient over a long-term hospital admission, with no known adverse outcomes. Though other interventions including salt supplementation, fortification of feeds and use of a PPI would have contributed to improving weight gain, without an effective method of PERT a patient with pancreatic insufficient CF would not be expected to consistently gain weight whilst receiving ≤100% EER, with no clinical symptoms of malabsorption. This technique is particularly useful for fine bore enteral tubes (<10-Fr) and/or with continuous feed regimens. It is recommended that the crushed enzyme beads are added to the formula 20 minutes prior to administration. For continuous feed infusions, it is preferable to add crushed enzymes to a maximum of 4 hours of formula at a time to be in line with the American Society for Parenteral and Enteral Nutrition recommendations on contamination and hang time [8]. When using this method, the dose of pancreatic enzyme required should be similar to the dose that would be calculated for oral administration as no loss in efficacy is expected.
Source: https://www.nature.com/articles/s41430-020-00727-y
0 Response to "Pancreatic Enzymes Continuous Tube Feeding How Often to Dose"
Post a Comment